Nov. 29, 2023
The National Institute of Health (NIH) has awarded Yunan Luo a grant for more than $1.8 million to use artificial intelligence (AI) to advance protein research.
New AI models produced through the grant will lead to new methods for the design and discovery of functional proteins. This could yield novel drugs and vaccines, personalized treatments against diseases, and other advances in biomedicine.
“This project provides a new paradigm to analyze proteins’ sequence-structure-function relationships using machine learning approaches,” said Luo, an assistant professor in Georgia Tech’s School of Computational Science and Engineering (CSE).
“We will develop new, ready-to-use computational models for domain scientists, like biologists and chemists. They can use our machine learning tools to guide scientific discovery in their research.”
Luo’s proposal improves on datasets spearheaded by AlphaFold and other recent breakthroughs. His AI algorithms would integrate these datasets and craft new models for practical application.
One of Luo’s goals is to develop machine learning methods that learn statistical representations from the data. This reveals relationships between proteins’ sequence, structure, and function. Scientists then could characterize how sequence and structure determine the function of a protein.
Next, Luo wants to make accurate and interpretable predictions about protein functions. His plan is to create biology-informed deep learning frameworks. These frameworks could make predictions about a protein’s function from knowledge of its sequence and structure. It can also account for variables like mutations.
In the end, Luo would have the data and tools to assist in the discovery of functional proteins. He will use these to build a computational platform of AI models, algorithms, and frameworks that ‘invent’ proteins. The platform figures the sequence and structure necessary to achieve a designed proteins desired functions and characteristics.
“My students play a very important part in this research because they are the driving force behind various aspects of this project at the intersection of computational science and protein biology,” Luo said.
“I think this project provides a unique opportunity to train our students in CSE to learn the real-world challenges facing scientific and engineering problems, and how to integrate computational methods to solve those problems.”
The $1.8 million grant is funded through the Maximizing Investigators’ Research Award (MIRA). The National Institute of General Medical Sciences (NIGMS) manages the MIRA program. NIGMS is one of 27 institutes and centers under NIH.
MIRA is oriented toward launching the research endeavors of young career faculty. The grant provides researchers with more stability and flexibility through five years of funding. This enhances scientific productivity and improves the chances for important breakthroughs.
Luo becomes the second School of CSE faculty to receive the MIRA grant. NIH awarded the grant to Xiuwei Zhang in 2021. Zhang is the J.Z. Liang Early-Career Assistant Professor in the School of CSE.
[Related: Award-winning Computer Models Propel Research in Cellular Differentiation]
“After NIH, of course, I first thanked my students because they laid the groundwork for what we seek to achieve in our grant proposal,” said Luo.
“I would like to thank my colleague, Xiuwei Zhang, for her mentorship in preparing the proposal. I also thank our school chair, Haesun Park, for her help and support while starting my career.”
News Contact
Bryant Wine, Communications Officer
bryant.wine@cc.gatech.edu
Oct. 05, 2023
The start of the fall semester can be busy for most Georgia Tech students, but this is especially true for Rafael Orozco. The Ph.D. student in Computational Science and Engineering (CSE) is part of a research group that presented at a major conference in August and is now preparing to host a research meeting in November.
We used the lull between events, research, and classes to meet with Orozco and learn more about his background and interests in this Meet CSE profile.
Student: Rafael Orozco
Research Interests: Medical Imaging; Seismic Imaging; Generative Models; Inverse Problems; Bayesian Inference; Uncertainty Quantification
Hometown: Sonora, Mexico
Tell us briefly about your educational background and how you came to Georgia Tech.
I studied in Mexico through high school. Then, I did my first two years of undergrad at the University of Arizona and transferred to Bucknell University. I was attracted to Georgia Tech’s CSE program because it is a unique combination of domain science and computer science. It feels like I am both a programmer and a scientist.
How did you first become interested in computer science and machine learning?
In high school, I saw a video demonstration of a genetic algorithm on the internet and became interested in the technology. My high school in Mexico did not have a computer science class, but a teacher mentored me and helped me compete at the Mexican Informatics Olympiad. When I started at Arizona, I researched the behavior of clouds from a Bayesian perspective. Since then, my research interests have always involved using Bayesian techniques to infer unknowns.
You mentioned your background a few times. Since it is National Hispanic Heritage Month, what does this observance mean to you?
I am quite proud to be a part of this group. In Mexico and the U.S., fellow Hispanics have supported me and my pursuits, so I know firsthand of their kindness and resourcefulness. I think that Hispanic people welcome others, celebrating the joy our culture brings, and they appreciate that our country uses the opportunity to reflect on Hispanic history.
You study in Professor Felix Herrmann’s Seismic Laboratory for Imaging and Modeling (SLIM) group. In your own words, what does this research group do?
We develop techniques and software for imaging Earth’s subsurface structures. These range from highly performant partial differential equation solvers to randomized numerical algebra to generative artificial intelligence (AI) models.
One of the driving goals of each software package we develop is that it needs to be scalable to real world applications. This entails imaging seismic areas that can be kilometers cubed in volume, represented typically by more than 100,000,000 simulation grid cells. In my medical applications, high-resolution images of human brains that can be resolved to less than half a millimeter.
The International Meeting for Applied Geoscience and Energy (IMAGE) is a recent conference where SLIM gave nine presentations. What research did you present here?
The challenge of applying machine learning to seismic imaging is that there are no examples of what the earth looks like. While making high quality reference images of human tissues for supervised machine learning is possible, no one can “cut open” the earth to understand exactly what it looks like.
To address this challenge, I presented an algorithm that combines generative AI with an unsupervised training objective. We essentially trick the generative model into outputting full earth models by making it blind to which part of the Earth we are asking for. This is like when you take an exam where only a few questions will be graded, but you don’t know which ones, so you answer all the questions just in case.
While seismic imaging is the basis of SLIM research, there are other applications for the group’s work. Can you discuss more about this?
The imaging techniques that the energy industry has been using for decades toward imaging Earth’s subsurface can be applied almost seamlessly to create medical images of human sub tissue.
Lately, we have been tackling the particularly difficult modality of using high frequency ultrasound to image through the human skull. In our recent paper, we are exploring a powerful combination between machine learning and physics-based methods that allows us to speed up imaging while adding uncertainty quantification.
We presented the work at this year’s MIDL conference (Medical Imaging with Deep Learning) in July. The medical community was excited with our preliminary results and gave me valuable feedback on how we can help bring this technique closer to clinical viability.
News Contact
Bryant Wine, Communications Officer
bryant.wine@cc.gatech.edu
Jun. 16, 2023
The discovery of nucleic acids is a recent event in the history of scientific phenomenon, and there is still much learn from the enigma that is genetic code.
Advances in computing techniques though have ushered in a new age of understanding the macromolecules that form life as we know it. Now, one Georgia Tech research group is receiving well-deserved accolades for their applications in data science and machine learning toward single-cell omics research.
Students studying under Xiuwei Zhang, an assistant professor in the School of Computational Science and Engineering (CSE), received awards in April at the Atlanta Workshop on Single-cell Omics (AWSOM 2023).
School of CSE Ph.D. student Ziqi Zhang received the best oral presentation award, while Mihir Birfna, an undergraduate student majoring in computer science, took the best poster prize.
Along with providing computational tools for biological researchers, the group’s papers presented at AWSOM 2023 could benefit populations of people as the research could lead to improved disease detection and prevention. They can also provide a better understanding of causes and treatments of cancer and new ability to accurately simulate cellular processes.
“I am extremely proud of the entire research group and very thankful of their work and our teamwork within our lab,” said Xiuwei Zhang. “These awards are encouraging because it means we are on the right track of developing something that will contribute both to the biology community and the computational community.”
Ziqi Zhang presented the group’s findings of their deep learning framework called scDisInFact, which can carry out multiple key single cell RNA-sequencing (scRNA-seq) tasks all at once and outperform current models that focus on the same tasks individually.
The group successfully tested scDisInFact on simulated and real Covid-19 datasets, demonstrating applicability in future studies of other diseases.
Bafna’s poster introduced CLARIFY, a tool that connects biochemical signals occurring within a cell and intercellular communication molecules. Previously, the inter- and intra-cell signaling were often studied separately due to the complexity of each problem.
Oncology is one field that stands to benefit from CLARIFY. CLARIFY helps to understand the interactions between tumor cells and immune cells in cancer microenvironments, which is crucial for enabling success of cancer immunotherapy.
At AWSOM 2023, the group presented a third paper on scMultiSim. This simulator generates data found in multi-modal single-cell experiments through modeling various biological factors underlying the generated data. It enables quantitative evaluations of a wide range of computational methods in single-cell genomics. That has been a challenging problem due to lack of ground truth information in biology, Xiuwei Zhang said.
“We want to answer certain basic questions in biology, like how did we get these different cell types like skin cells, bone cells, and blood cells,” she said. “If we understand how things work in normal and healthy cells, and compare that to the data of diseased cells, then we can find the key differences of those two and locate the genes, proteins, and other molecules that cause problems.”
Xiuwei Zhang’s group specializes in applying machine learning and optimization skills in analysis of single-cell omics data and scRNA-seq methods. Their main interest area is studying mechanisms of cellular differentiation— the process when young, immature cells mature and take on functional characteristics.
A growing, yet effective approach to research in molecular biology, scRNA-seq gives insight of existence and behavior of different types of cells. This helps researchers better understand genetic disorders, detect mechanisms that cause tumors and cancer, and develop new treatments, cures, and drugs.
If microenvironments filled with various macromolecules and genetic material are considered datasets, the need for researchers like Xiuwei Zhang and her group is obvious. These massive, complex datasets present both challenges and opportunities for the group experienced in computational and biological research.
Collaborating authors include School of CSE Ph.D. students Hechen Li and Michael Squires, School of Electrical and Computer Engineering Ph.D. student Xinye Zhao, Wallace H. Coulter Department of Biomedical Engineering Associate Professor Peng Qiu, and Xi Chen, an assistant professor at Southern University of Science and Technology in Shenzhen, China.
The group’s presentations at AWSOM 2023 exhibited how their work makes progress in biomedical research, as well as advancing scientific computing methods in data science, machine learning, and simulation.
scDisInFact is an optimization tool that can perform batch effect removal, condition-associated key gene detection, and perturbation, which is made possible by considering major variation factors in the data. Without considering all these factors, current models can only do these tasks individually, but scDisInFact can do each task better and all at the same time.
CLARIFY delves into how cells employ genetic material to communicate internally, using gene regulatory networks (GRNs) and externally, called cell-cell interactions (CCIs). Many computational methods can infer GRNs and inference methods have been proposed for CCIs, but until CLARIFY, no method existed to infer GRNs and CCIs in the same model.
scMultiSim simulations perform closer to real-world conditions than current simulators that model only one or two biological factors. This helps researchers more realistically test their computational methods, which can guide directions for future method development.
Whether they be computer scientists, biologists, or non-academics alike, the advantage of interdisciplinary and collaborative research, like Xiuwei Zhang’s group, is its wide-reaching impact that advances technology to improve the human condition.
“We’re exploring the possibilities that can be realized by advanced computational methods combined with cutting edge biotechnology,” said Xiuwei Zhang. “Since biotechnology keeps evolving very fast and we want to help push this even further by developing computational methods, together we will propel science forward.”
News Contact
Bryant Wine, Communications Officer
bryant.wine@cc.gatech.edu
Apr. 06, 2023
Professor Omer Inan is set to take the stage at the upcoming TEDxAtlanta 2023: We Rise event on May 19.
As the Linda J. and Mark C. Smith Chair in bioscience and bioengineering in Tech’s School of Electrical and Computer Engineering (ECE), Inan designs clinically relevant medical devices and systems and translates them from the lab to patient care applications. In his talk, Inan will be discussing his groundbreaking research on wearable healthcare technologies and the potential they hold for revolutionizing the field.
Inan is a member of the prestigious Medical and Biological Engineering (AIMBE) College of Fellows (elected in 2022) for his “outstanding contributions to the non-invasive assessment of the mechanical aspects of cardiovascular health and performance using wearable devices.” Additional achievements include an Academy Award for Technical Achievement from The Academy of Motion Picture Arts and Sciences (The Oscars, 2021), the Georgia Power Professor of Excellence for the College of Engineering (2019), and the National Science Foundation Faculty Early Career Development Program award (NSF CAREER, 2018).
TEDxAtlanta 2023: WE RISE brings together an impressive group of participants from diverse backgrounds, experiences, and perspectives. The speakers include entrepreneurs, activists, educators, artists, scientists, and many other changemakers who have risen above challenges to make a positive impact on the world.
The event's participants will share their stories and insights on how they have overcome adversity, embraced innovation, and challenged the status quo to make a difference in their communities and beyond. Through their talks, they will inspire and empower attendees to rise above their own challenges and take action towards creating a better future for all.
TEDxAtlanta 2023: WE RISE will take place on Friday, May 19 from 9 a.m. – 6:30 p.m. at the Rialto Center for the Arts (80 Forsyth Street Northwest Atlanta, GA 30303). Learn more and purchase tickets at tedxatlanta.com.
News Contact
Dan Watson
Jan. 03, 2023
Though it is a cornerstone of virtually every process that occurs in living organisms, the proper folding and transport of biological proteins is a notoriously difficult and time-consuming process to experimentally study.
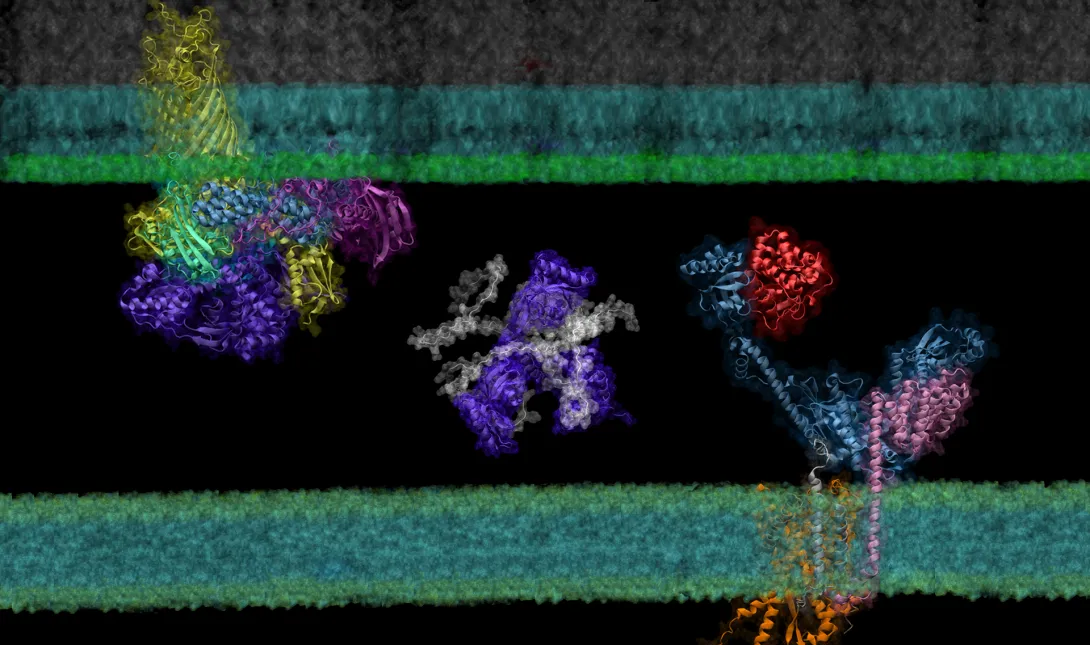
In a new paper published in eLife, researchers in the School of Biological Sciences and the School of Computer Science have shown that AF2Complex may be able to lend a hand.
Building on the models of DeepMind’s AlphaFold 2, a machine learning tool able to predict the detailed three-dimensional structures of individual proteins, AF2Complex — short for AlphaFold 2 Complex — is a deep learning tool designed to predict the physical interactions of multiple proteins. With these predictions, AF2Complex is able to calculate which proteins are likely to interact with each other to form functional complexes in unprecedented detail.
“We essentially conduct computational experiments that try to figure out the atomic details of supercomplexes (large interacting groups of proteins) important to biological functions,” explained Jeffrey Skolnick, Regents’ Professor and Mary and Maisie Gibson Chair in the School of Biological Sciences, and one of the corresponding authors of the study. With AF2Complex, which was developed last year by the same research team, it’s “like using a computational microscope powered by deep learning and supercomputing.”
In their latest study, the researchers used this ‘computational microscope’ to examine a complicated protein synthesis and transport pathway, hoping to clarify how proteins in the pathway interact to ultimately transport a newly synthesized protein from the interior to the outer membrane of the bacteria — and identify players that experiments might have missed. Insights into this pathway may identify new targets for antibiotic and therapeutic design while providing a foundation for using AF2Complex to computationally expedite this type of biology research as a whole.
Computing complexes
Created by London-based artificial intelligence lab DeepMind, AlphaFold 2 is a deep learning tool able to generate accurate predictions about the three-dimensional structure of single proteins using just their building blocks, amino acids. Taking things a step further, AF2Complex uses these structures to predict the likelihood that proteins are able to interact to form a functional complex, what aspects of each structure are the likely interaction sites, and even what protein complexes are likely to pair up to create even larger functional groups called supercomplexes.
“The successful development of AF2Complex earlier this year makes us believe that this approach has tremendous potential in identifying and characterizing the set of protein-protein interactions important to life,” shared Mu Gao, a senior research scientist at Georgia Tech. “To further convince the broad molecular biology community, we [had to] demonstrate it with a more convincing, high impact application.”
The researchers chose to apply AF2Complex to a pathway in Escherichia coli (E. coli), a model organism in life sciences research commonly used for experimental DNA manipulation and protein production due to its relative simplicity and fast growth.
To demonstrate the tool’s power, the team examined the synthesis and transport of proteins that are essential for exchanging nutrients and responding to environmental stressors: outer membrane proteins, or OMPs for short. These proteins reside on the outermost membrane of gram-negative bacteria, a large family of bacteria characterized by the presence of inner and outer membranes, like E. coli. However, the proteins are created inside the cell and must be transported to their final destinations.
“After more than two decades of experimental studies, researchers have identified some of the protein complexes of key players, but certainly not all of them,” Gao explained. AF2Complex “could enable us to discover some novel and interesting features of the OMP biogenesis pathway that were missed in previous experimental studies.”
New insights
Using the Summit supercomputer at the Oak Ridge National Laboratory, the team, which included computer science undergraduate Davi Nakajima An, put AF2Complex to the test. They compared a few proteins known to be important in the synthesis and transport of OMPs to roughly 1,500 other proteins — all of the known proteins in E. coli’s cell envelope — to see which pairs the tool computed as most likely to interact, and which of those pairs were likely to form supercomplexes.
To determine if AF2Complex’s predictions were correct, the researchers compared the tool’s predictions to known experimental data. “Encouragingly,” said Skolnick, “among the top hits from computational screening, we found previously known interacting partners.” Even within those protein pairs known to interact, AF2Complex was able to highlight structural details of those interactions that explain data from previous experiments, lending additional confidence to the tool’s accuracy.
In addition to known interactions, AF2Complex predicted several unknown pairs. Digging further into these unexpected partners revealed details on what aspects of the pairs might interact to form larger groups of functional proteins, likely active configurations of complexes that have previously eluded experimentalists, and new potential mechanisms for how OMPs are synthesized and transported.
“Since the outer membrane pathway is both vital and unique to gram-negative bacteria, the key proteins involved in this pathway could be novel targets for new antibiotics,” said Skolnick. “As such, our work that provides molecular insights about these new drug targets might be valuable to new therapeutic design.”
Beyond this pathway, the researchers are hopeful that AF2Complex could mean big things for biology research.
“Unlike predicting structures of a single protein sequence, predicting the structural model of a supercomplex can be very complicated, especially when the components or stoichiometry of the complex is unknown,” Gao noted. “In this regard, AF2Complex could be a new computational tool for biologists to conduct trial experiments of different combinations of proteins,” potentially expediting and increasing the efficiency of this type of biology research as a whole.
AF2Complex is an open-source tool available to the public and can be downloaded here.
This work was supported in part by the DOE Office of Science, Office of Biological and Environmental Research (DOE DE-SC0021303) and the Division of General Medical Sciences of the National Institute Health (NIH R35GM118039). DOI: https://doi.org/10.7554
News Contact
Writer: Audra Davidson
Communications Officer
College of Sciences at Georgia Tech
Editor: Jess Hunt-Ralston
Director of Communications
College of Sciences at Georgia Tech
Nov. 30, 2020
When one or more coronavirus vaccines receives FDA emergency use authorization, it will launch a public health and logistics initiative unlike any in U.S. history.
Hundreds of millions of doses will have to distributed nationwide and kept cold until healthcare professionals can administer not one, but two doses to each person. And enough skeptical members of the population will have to be persuaded to receive the vaccine to slow virus transmission.
Beyond those challenges, the distribution effort will have to adapt to unexpected and uneven demand; accommodate recipients who may not return on time for a second dose; train hundreds of thousands of staff from clinics, pharmacies, doctor’s offices, and hospitals; prioritize serving high-risk groups first while encouraging others to wait — all while under tremendous pressure to get the much-anticipated vaccines into use as case counts and the death toll continue rising.
“Time is of the essence because the virus is already so widespread,” said Pinar Keskinocak, the William W. George Chair and professor in the H. Milton Stewart School of Industrial and Systems Engineering (ISyE) and director of the Center for Health and Humanitarian Systems at the Georgia Institute of Technology. “With the pressure on our timeline, knowledge of how quickly the disease is spreading, and the broad U.S. and global need, I can’t think of a comparable public health initiative that has ever been undertaken.”
Shipping and Keeping Hundreds of Millions of Doses Cold
Three vaccines, produced by Moderna, Pfizer and its German partner BioNTech, and Oxford-AstraZeneca, are expected to be available first. The Pfizer-BioNTech vaccine will need to be kept ultra-cold — minus 94 degrees Fahrenheit — on its journey to individual Americans. The Moderna drug won’t have such demanding conditions, but both it and the Pfizer vaccine will tax the existing “cold chain” that keeps vaccines and other temperature-sensitive products in a narrow range of conditions during transport and storage.
The Oxford-AstraZeneca vaccine will have much less stringent requirements and faster ramp-up in capacity, though early testing suggests its efficacy may be lower than the others. That will create tradeoffs between efficacy versus access and speed in distribution.
Plans already exist to get the vaccines from manufacturers to the states, each of which has developed its own distribution plan. Keskinocak worries mostly about “last mile” plans — getting the vaccines to where they will be injected — and getting individuals to those locations.
“Access is going to be a challenge,” she said. “You may be able to get it to locations where it can be distributed, but you have to make sure the people who really need the vaccine can easily access those locations.”
Cold chain transportation, tracking, tracing, and storage already exist in most areas, but refrigeration could be challenging for rural areas that may be at the end of the chain, especially for the vaccine requiring very cold temperatures beyond the capability of freezers found in most doctor’s offices and clinics. And cold can sometimes be too cold, Keskinocak said.
“We often think about keeping it cold, but sometimes it may be too cold, which is not good. It’s not just whether the temperature exceeded the required level, but also whether it went below that. It is important to keep the vaccine exactly at the required temperature level.”
Pfizer has developed a shipping container that includes a temperature tracking device — and 50 pounds of dry ice to maintain the right temperature during transit. Because it is contained in small vials and the liquid vaccine is diluted for use, the overall volume being shipped will be relatively small, limiting the number of packages that will be moved and stored, Keskinocak noted.
Ultimately, the cold chain may play a significant role in vaccine effectiveness. Currently, the vaccines being produced by Pfizer/BioNTech and Moderna are reported to have a higher efficacy than the Oxford-AstraZeneca vaccine — but only if they can be maintained at the proper temperatures. The timing, magnitude, and duration of temperature fluctuations during transport and before administration could affect that in ways that may be difficult to assess.
“Our current modeling shows that a vaccine that is less effective but that can be distributed more quickly and more widely might work better in some settings than a more effective vaccine, thereby reducing the total number of infections in the population,” Keskinocak said.
If You Build It, Will They Come?
Expectations are that the nation is hungry for a vaccine to escape the horrors of Covid-19. But a recent Gallup survey shows that only 58% of respondents said they planned to receive the vaccine when it becomes available. Boosting that percentage will require a massive communications effort to overcome vaccine reluctance and concerns fueled by the uneven nature of the U.S. pandemic response.
“If we can get the vaccine to locations where people can access it, and we have the necessary syringes, supplies, and PPE, as well as the healthcare staff to administer the injections, it’s not clear that people will come to receive it in large enough numbers,” Keskinocak said. “That’s one major component missing from a lot of the plans that I see at the state level.”
The communications program will have to run in parallel to the vaccine distribution, and they have to be coordinated so that supply meets demand.
“Public health communication and dissemination of information at the right time and in the right language is going to be at least as important and challenging as the logistics of distributing the vaccine,” Keskinocak said. “Communication is going to shape demand to a large extent. If one is more effective than the other, we will have a mismatch between demand and supply.”
Different demographic populations have different levels of trust for medicine in general and vaccines in particular, she said, so communications campaigns will have to focus on issues of concern to those groups. Unexpected variations in vaccine demand caused by these concerns could also create logistical uncertainties.
“We can try to forecast demand, and ship supplies to those locations,” she said. “But historically, we have seen that demand can exceed supply in one location while inventory builds up in another location. We need to avoid this situation of unmet demand and unused vaccine.”
Another issue will be the two doses necessary for the vaccine. The second dose must be received within a narrow range of time for the two-dose vaccine to be effective. Should a second dose be reserved for every person receiving a first dose, or should the goal be to get as many doses out as possible?
“Some people may never show up to be vaccinated, while others will receive the first dose, but may not come back for the second dose,” she said.
Getting the Program Started
The first available doses will likely go to healthcare workers and first responders who are on the front lines of battling Covid-19. That is expected to be the easier part of vaccination logistics, and the lessons learned there should help with the much more massive vaccination campaign for high-risk individuals and the general public.
As vaccine production and distribution capacity ramp up, other groups will be next in line. While distributing small batches as manufacturers produce it can create some supply challenges, that also allows the system to more easily adjust to unexpected demand.
Even though distributing and administering vaccines is something the U.S. healthcare system does routinely, the size and timeline of this project are unprecedented, Keskinocak noted.
Beyond the logistical and communications needs, the vaccination program will also have a strong information technology component. Administration will likely be by appointment, and each injection will have to be reported to a vaccine registry to provide a record of which vaccines people have received and when.
Vaccinating People Who May Already Be Immune
It’s estimated that the number of reported Covid-19 cases may be just 10% of the actual number of infections in the U.S. Assuming recovery from the virus confers immunity for some period of time means there may be quite a few people who don’t actually need the vaccine right away to be protected. But there are currently no plans to determine whether recipients are already immune before they receive the vaccine.
“There are a lot of people out there who have some level of immunity to the coronavirus,” Keskinocak said. “The plans I’ve seen don’t include the serological testing that would be needed to identify people with some level of immunity, which could be around 30% of the population by the time the vaccine gets out to the general public.”
Testing for immune antibodies could be done ahead of the vaccination program, but that would create an extra step in a process that is already quite complicated. Healthcare systems such as the U.S. Department of Veterans Affairs or certain private insurance plans could include that step, especially if vaccine supplies lag behind demand.
“The big complexity is timing,” she said. “Once vaccines become available, you’ll want to deliver them as quickly as possible to as many people as possible in a very short time frame.”
Annual vaccination campaigns for the seasonal flu set ambitious goals for the population levels they want to reach, but the time challenges will be much greater for the coronavirus vaccine.
“The seasonal flu vaccine becomes available months before the virus spreads broadly, so we have quite a bit of time to administer it before we get into the peak of the flu season,” she said. “We have been in the midst of the Covid-19 pandemic for several months now. We are really late in the game, so we don’t have the luxury of time.”
Keskinocak is cautiously optimistic that the challenges will ultimately be addressed.
“There are certainly still lots of unknowns,” she said. “But the state plans I have seen look reasonable from a supply chain standpoint. Some of the decisions will be made once the states receive the vaccine, and exactly how they do it will be somewhat up to the local jurisdictions. There are still many things that need to be decided to make this unprecedented initiative live up to its goals.”
Research News
Georgia Institute of Technology
177 North Avenue
Atlanta, Georgia 30332-0181 USA
Media Relations Contact: John Toon (404-894-6986) (jtoon@gatech.edu)
Writer: John Toon
News Contact
John Toon
Research News
(404-894-6986)
Aug. 06, 2020
BioFabUSA, a Department of Defense-funded Manufacturing Innovation Institute within the Manufacturing USA network, has awarded the Georgia Institute of Technology and industry partner, Akron Biotech, a project titled, “Supply Chain and Process Modeling Algorithms, Methods, and Tools for Tissue Manufacturing and Distribution”. This project will address significant national supply chain issues related to distributing tissue engineered medical products (TEMPs) to U.S. patients in need.
The project aims to create the first simulation-based supply chain model for the rapidly evolving and future facing TEMPs industry, to minimize manufacturing and logistics costs and risks, incorporate Department of Defense (DOD) and other stakeholders’ perspectives into supply chain modeling, inform standards development, and support workforce development.
“Having a supply chain model will be instrumental in helping new and existing companies plan for the most efficient process flows, resource usage, and cost savings,” said Stephanie Robichaud, technical project manager with the Advanced Regenerative Manufacturing Institute. “Many startup companies do not realize some of the intricacies in managing their supply chain and many established companies realize the importance of it after experiencing inefficiencies. Having a model that these companies can use will help advance the field of tissue engineering as they plan for scale-up.”
According to Ben Wang, executive director of the Georgia Tech Manufacturing Institute (GTMI) and professor in the Stewart School of Industrial and Systems Engineering, “hundreds if not thousands of patients are waiting for tissues and organs in order to have a normal healthy life. Our project is a bold initiative to democratize distribution of replacement tissues and organs by streamlining national supply chains. This project will develop simulation-based tools to enhance the efficiency and resilience of the TEMPs supply chain, making these personalized medicines more affordable and more accessible.”
The growth of the TEMP industry is going to change the supply chain of medical tissues disruptively. To embrace this change, a system-level decision support tool is essential for adopting more cost-effective manufacturing processes and making better investment decisions. To ensure successful commercialization and adoption of this new supply chain decision support tool, the project team will engage multiple stakeholders including DOD, government, regulatory bodies, standards setting organizations, patients, industry, academia, policy experts, education and workforce development experts.
Georgia Tech project leads include Ben Wang, Ph.D., Chelsea C. White III, Ph.D, and Kan Wang, Ph.D. Ben Wang is Gwaltney Chair in Manufacturing Systems, professor in the Stewart School of Industrial & Systems Engineering and School of Materials Science and Engineering at Georgia Tech. In addition, he serves as executive director of the Georgia Tech Manufacturing Institute (GTMI). Chelsea C. White III is the Schneider National Chair in Transportation and Logistics and professor in the H. Milton Stewart School of Industrial and Systems Engineering at Georgia Tech. Kan Wang is lead researcher of additive manufacturing in the Bio-Engineering Research Laboratory at GTMI.
Leading the project for Akron Biotech is Ezequiel Zylberberg, Ph.D, who is vice president of product development and planning. According to Ezequiel, “the future of regenerative medicine depends on more than our ability to address the scientific challenges of generating the next generation of advanced therapies. Advancing these novel treatments in a way that is scalable will require significant advances in manufacturing innovation. We are eager to collaborate with our colleagues at Georgia Tech, at BioFab USA, and throughout the regenerative medicine industry to confront the challenge of scalability and supply chain resilience through this modelling effort.”
About the Georgia Institute of Technology
The Georgia Institute of Technology, also known as Georgia Tech, is one of the nation’s leading research universities — a university that embraces change while continually Creating the Next. The next generation of leaders. The next breakthrough startup company. The next lifesaving medical treatment.
Georgia Tech provides a focused, technologically based education to more than 36,000 undergraduate and graduate students. The Institute has many nationally recognized programs, all top-ranked by peers and publications alike, and is ranked among the nation’s top five public universities by U.S. News & World Report. It offers degrees through the Colleges of Computing, Design, Engineering, Sciences, the Scheller College of Business, and the Ivan Allen College of Liberal Arts. As a leading technological university, Georgia Tech has more than 100 centers focused on interdisciplinary research that consistently contribute vital research and innovation to American government, industry, and business. https://www.gatech.edu/
About Akron Biotech
Akron is a leading materials manufacturer and services provider to the regenerative medicine industry, accelerating the development and commercialization of advanced therapies. Founded in 2006, Akron is an ISO 13485-certified company that operates in line with cGMPs and international standards, enabling advanced therapy developers to de-risk their supply chains and facilitate regulatory approval. The company's unique business model emphasizes knowledge, flexibility and unparalleled service—from development through to commercialization. For more information, please visit www.akronbiotech.com.
About BioFabUSA
BioFabUSA, is a DOD-funded Manufacturing USA Innovation Institute (MII) sustained by the Advanced Regenerative Manufacturing Institute (ARMI) is a non-profit organization located in Manchester, New Hampshire. ARMI's mission is to make practical the scalable, consistent, cost-effective manufacturing of tissue engineered medical products and tissue-related technologies, to benefit existing industries and grow new ones. https://www.armiusa.org/
Georgia Tech Manufacturing Institute
813 Ferst Drive, NW
Atlanta, GA 30332 USA
Media Relations Contact: Walter Rich (walter.rich@research.gatech.edu)
News Contact
Jul. 28, 2020
Personal initiatives by a pediatrician and by researchers to make face shields for medical workers have transformed into an industry collaboration that by June had delivered 1.8 million shields to hospitals and other organizations around the country with plans to produce 2.5 million all total. A $2 million donation from Aflac Incorporated for personal protective equipment (PPE) financed the bulk of the shields.
To make it happen, a team of researchers and industry partners convened at the Global Center for Medical Innovation (GCMI), a Georgia Tech-affiliated nonprofit that guides new experimental medical solutions to market. The group combined the physician’s vision with the researchers’ original designs, adjusted them to pass FDA emergency guidelines, and then coordinated mass production and distribution.
A physician’s wisdom
The project grew wings in mid-March, after Dr. Joanna Newton became concerned that the nationwide shortage of PPE was leaving healthcare workers across the country vulnerable. Newton is a physician specializing in improving healthcare safety through technology at Children’s Healthcare of Atlanta, and she was already collaborating with Georgia Tech on other projects.
She grabbed the phone to leverage the connection.
“I called Sherry Farrugia to tell her about my idea to 3D-print PPE. We needed to quickly find a solution for the PPE shortage around the country, and I knew we had the right team here in Atlanta to help,” said Newton, a pediatric hematologist/oncologist at the Aflac Cancer and Blood Disorders Center of Children’s.
“The situation was urgent, and I knew who would have the right expertise to get this done,” said Farrugia, chief operating officer and strategy officer of Children’s Healthcare of Atlanta Pediatric Technology Center, which is part of Georgia Tech.
Farrugia had Newton present her idea at GCMI to researchers, advisors, and industry partners who immediately put together a team to address the need for face shields to protect healthcare workers from droplets containing the coronavirus. She also discussed the need with Devesh Ranjan, associate chair of the George W. Woodruff School of Mechanical Engineering, who suggested connecting the effort to a parallel initiative in that school.
Bringing in engineers
At the same time, along with Ranjan, Sam Graham, chair of the George W. Woodruff School of Mechanical Engineering, and Susan Margulies, chair of the Wallace H. Coulter Department of Biomedical Engineering, were coordinating efforts across campus to develop various medical devices in response to the pandemic. Graham, Margulies, and Ranjan quickly connected GCMI with Christopher Saldana and Saad Bhamla, faculty members in Georgia Tech’s College of Engineering, who were leading an simultaneous effort to address the face shield problem with their students using rapid fabrication techniques like 3-D printing, laser cutting, and waterjet cutting.
“The Georgia Tech mechanical engineering team used rapid fabrication equipment and quickly produced multiple face shield designs that could be manufactured in high volumes for the rapid response environment that Covid-19 required,” Saldana said.
Making a few thousand shields in a lab had likely already saved lives, but the Georgia Tech researchers and GCMI put their designs on the internet, where they have been downloaded thousands of times by organizations manufacturing them around the world. And the manufacturing partners they engaged have been turning out hundreds of thousands of shields to save many more lives.
“You may need 45 minutes for a headband with a 3D printer, but manufacturers turn out six of them every 19 seconds. Then making a million face shields becomes a real possibility,” said Mike Fisher, who leads product development at GCMI.
GCMI opened a GoFundMe page, which brought in $20,000, and then engaged their first manufacturing partner, Delta Air Lines.
A manufacturing explosion
“Delta converted one of their groups from manufacturing airplane interiors to doing the face shields. They started off by manufacturing 6,000 shields, and that got the momentum going,” Leiter said. “Two thousand shields went to Mount Sinai Hospital in New York; 2,000 went to Piedmont Healthcare in Atlanta; and 2,000 went to Children’s Healthcare of Atlanta.”
Things began to snowball.
Graham engaged Siemens Industries to fulfill a face shield order from the Georgia Emergency Management Agency (GEMA) for distribution in Georgia. Partners from ExxonMobil began looking for more potential manufacturers. And Aflac contacted Children’s looking for worthy Covid-19 related efforts to support.
“We asked for a donation of $500,000 for manufacturers to retool their operations. Aflac made a gift of $2 million to GCMI to promote the production of PPE,” Farrugia said. “We were able to buy tooling for an automotive plastics manufacturer called Quality Model in South Carolina, and they have made over 750,000 face shields so far.”
GCMI won a bid from the Federal Emergency Management Agency (FEMA) for 1,141,600 face shields, which are being made by Quality Model, where ExxonMobil helped rearrange production lines for shields.
Siemens made an additional 100,000 shields from Aflac’s gift, which is also being used to purchase existing PPE to donate to healthcare workers. Kia Motors quickly produced an initial 15,000 shields, which the company financed itself.
“Kia got the open source design from the Georgia Tech website and ran with it on their own,” Saldana said.
These partners are delivering the following number of shields: Quality Model, 1,251,600; Kia Motors, 300,000; Siemens Industries, 205,000; Delta Air Lines, 106,100; Georgia Tech, 20,000; and EIS, 15,000. And more are still to come.
The shields went across the country, from hospitals in New York City to Prisma Health in South Carolina, to nursing homes in the Pensacola area, and to rural Louisiana and Mississippi, Leiter said.
Thanks in large part to Aflac’s gift, GCMI and Farrugia are coordinating with partners, including Georgia Tech engineers, to produce N95 masks, hospital gowns, and hand sanitizer, all redesigned for the Covid-19 age.
Research News
Georgia Institute of Technology
177 North Avenue
Atlanta, Georgia 30332-0181 USA
Media Relations Assistance: John Toon (404-894-6986) (jtoon@gatech.edu).
Writer: Ben Brumfield
News Contact
John Toon
Research News
(404) 894-6986
Apr. 22, 2020
Gaps in the supply of coronavirus tests are propelling initiatives to fill them across the country. At the Georgia Institute of Technology, bioscience researchers are burning the midnight oil to produce key components for tests in the state of Georgia.
The goal is to supply a broad initiative by the governor’s office involving multiple universities and partners to rapidly produce and administer more tests. At least 35 volunteers at Georgia Tech, while adhering to social distancing, are reorienting labs normally used for scientific discovery to do larger-scale production of biochemical components.
“We are inventing new ways of doing things like an electronic buddy system so people can be alone – but not alone – while they work in the lab. The technical part is actually the easiest. The logistics of testing, data security, and regulatory considerations – those things are more challenging,” said Loren Williams, a professor in Georgia Tech’s School of Chemistry and Biochemistry.
Williams and the researchers are supporting Georgia Governor Brian Kemp’s COVID-19 State Lab Surge Capacity Task Force, which is a project managed through the Georgia Tech Research Institute (GTRI). GTRI is also leading the coordination and integration of data management across the lab surge effort.
“We are providing technical and project management of the effort which is focused on increasing the state’s ability to expand testing beyond current limitations,” said Mike Shannon, GTRI’s lead in the project and a principal research engineer at GTRI.
Exoplanets and coronavirus
The science behind coronavirus testing is complementary to the researchers’ usual work. That includes understanding proteins associated with glaucoma, figuring out how RNA and DNA evolved in the first place, or whether ribosomes – lumps of RNA and protein key to translating genetic code into life – may exist on exoplanets.
Williams’ research team studies the last topic, and some of their work is related to the core of coronavirus testing, a chemical reaction that amplifies the virus’ genetic fingerprint. It is called a reverse transcription polymerase chain reaction (RT-PCR), and it transcribes trace amounts of coronavirus’ RNA code into ample amounts of corresponding DNA in the lab for easy analysis.
“His lab members are very familiar with RT-PCR, and when the lack of tests became apparent, they swung into action. The group grew from there, based on the technical needs for the project,” said Raquel Lieberman, another leading scientist in the effort and also a professor in Georgia Tech’s School of Chemistry and Biochemistry.
“Every day, very talented, hardworking people with perfect skill sets come out of the woodwork and ask to help,” Williams said.
The group has teams that engineer the production of enzymes or other chemicals needed for RT-PCR to work: Two central enzymes are reverse transcriptase, which converts RNA to DNA and Taq polymerase, which rapidly replicates DNA. Another important component is ribonuclease inhibitor, which slows coronavirus RNA decay.
Global COVID allies
Other researchers develop processes for mass production or implementation of COVID-19 safety procedures; the list goes on. Some colleagues telework; others work in labs but spaced far from each other while they wear masks.
“The group is planning to produce enough enzyme components for hundreds of tests per day,” said Vinayah Agarwal, an assistant professor in Georgia Tech’s School of Chemistry and Biochemistry and School of Biological Sciences. “Using these components, we will also build cheaper and more robust testing kits going forward.”
Instructions already exist for some of the ingredients for the test, but they are not readily available because the rights to them are exclusive.
“Intellectual property and other proprietary issues hinder our effort,” Lieberman said. “But we have received help from scientists all over the world to piece together protocols on how to make what we need.”
The state wants to increase current testing capacities by 3,000 more tests per day. The task force also includes teams from Augusta University Health System, Georgia State University, Emory University, University of Georgia, and the Georgia Public Health Laboratory. The task force lead is Captain Kevin Caspary who is with the Georgia National Guard.
Raw footage and images as press handouts for journalists. (No commercial or personal use):
https://www.dropbox.com/sh/f2wc2i74lz1lffl/AADLJ8dQnZMr4uEDxAiIMusoa?dl=0
Also read this: Interactive COVID-19 tool shows the importance of staying at home
External News Coverage:
NPR - Sun Rays, Disinfectants And False Hopes: Misinformation Litters The Road To Reopening
News-Medical.Net - Georgia Tech researchers create key components for COVID-19 tests
Georgia Tech News Center- A New Normal: Researchers Across Georgia Tech Rally to Fight COVID-19
Here's how to subscribe to our free science and technology email newsletter
Writer & Media Representative: Ben Brumfield (404-272-2780), email: ben.brumfield@comm.gatech.edu
Georgia Institute of Technology
Apr. 15, 2020
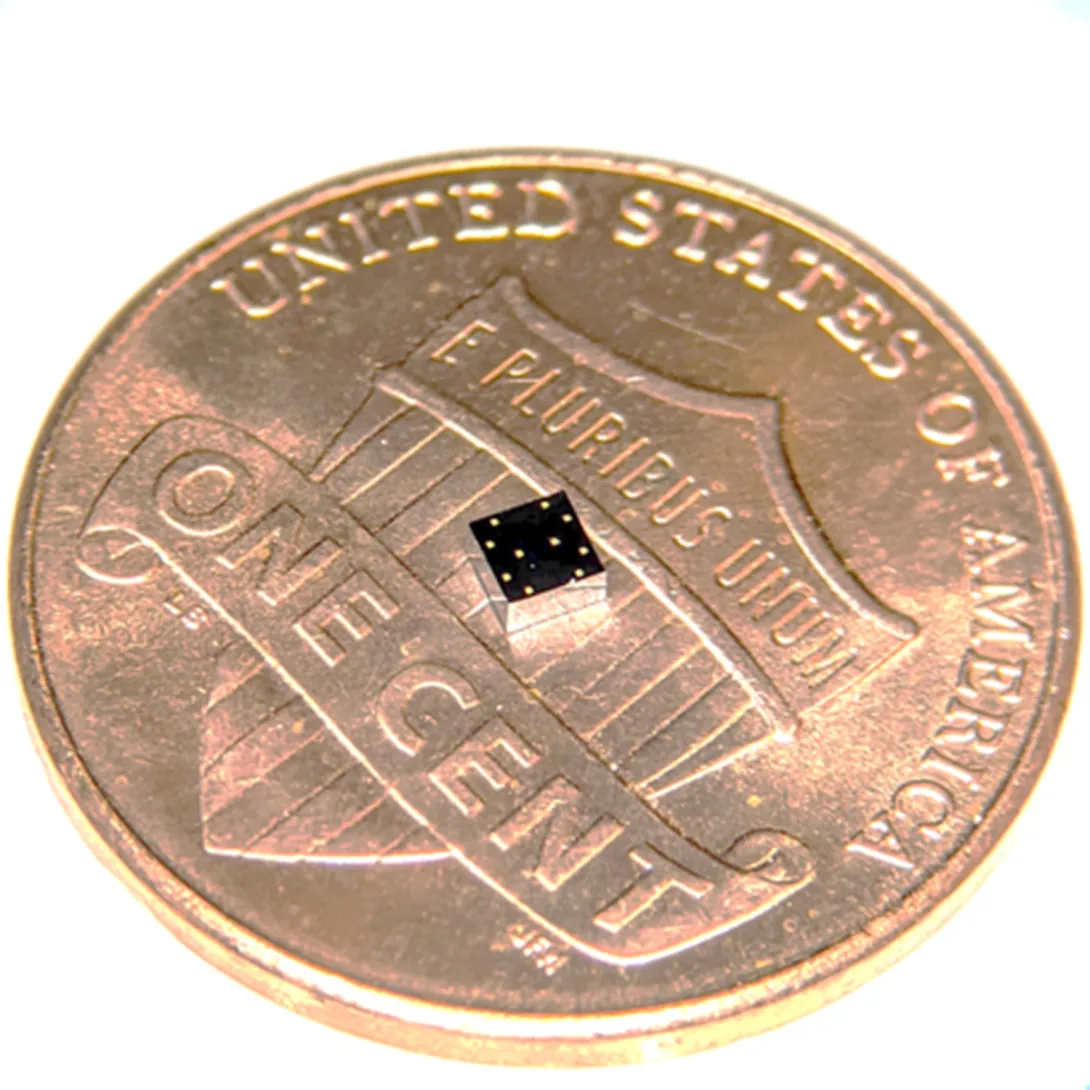
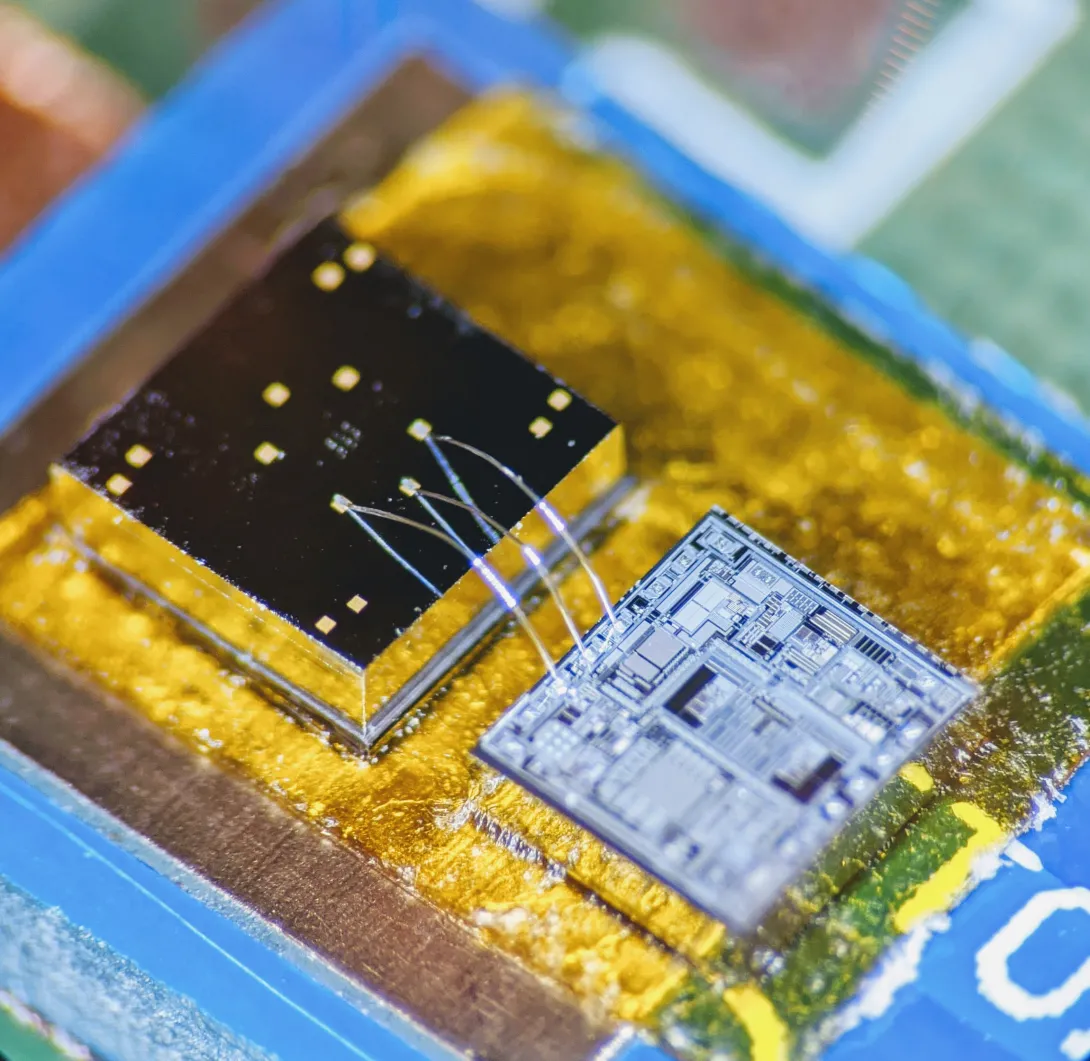
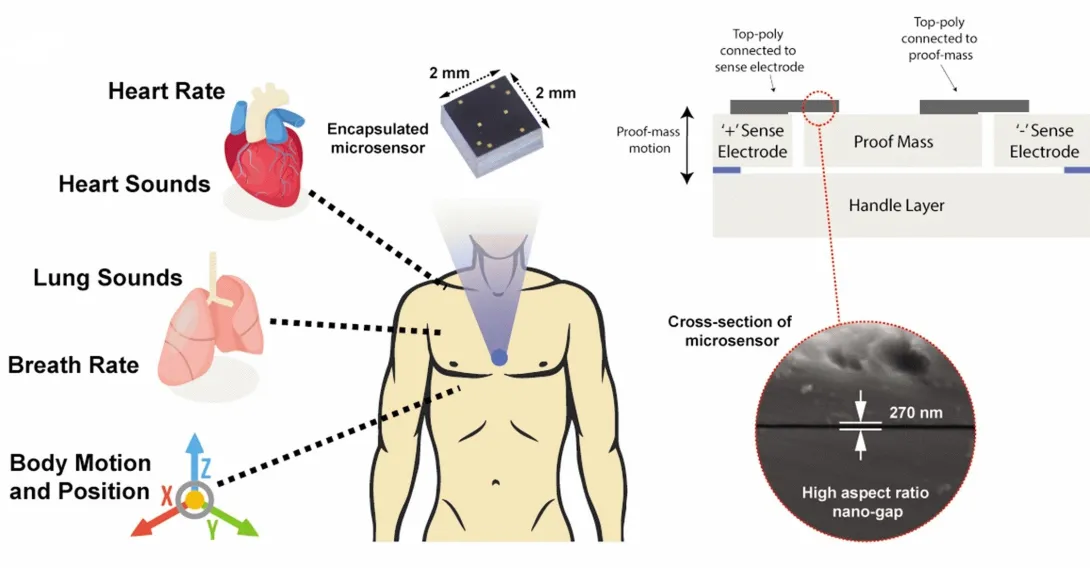
During a stroll, a woman’s breathing becomes a slight bit shallower, and a monitor in her clothing alerts her to get a telemedicine check-up. A new study details how a sensor chip smaller than a ladybug records multiple lung and heart signals along with body movements and could enable such a future socially distanced health monitor.
The core mechanism of the chip developed by researchers at the Georgia Institute of Technology involves two finely manufactured layers of silicon, which overlay each other separated by the space of 270 nanometers – about 0.000001 inches. They carry a minute voltage.
Vibrations from bodily motions and sounds put part of the chip in very slight motion, making the voltage flux, thus creating readable electronic outputs. In human testing, the chip has recorded a variety of signals from the mechanical workings of the lungs and the heart with clarity, signals that often escape meaningful detection by current medical technology.
“Right now, medicine looks to EKGs (electrocardiograms) for information on the heart, but EKGs only measure electrical impulses. The heart is a mechanical system with muscles pumping and valves opening and shutting, and it sends out a signature of sounds and motions, which an EKG does not detect. EKGs also say nothing about lung function,” said Farrokh Ayazi, Ken Byers Professor in Georgia Tech’s School of Electrical and Computer Engineering.
Stethoscope-accelerometer combo
The chip, which acts as an advanced electronic stethoscope and accelerometer in one, is aptly called an accelerometer contact microphone. It detects vibrations that enter the chip from inside the body while keeping out distracting noise from outside the body's core like airborne sounds
“If it rubs on my skin or shirt, it doesn’t hear the friction, but the device is very sensitive to sounds coming at it from inside the body, so it picks up useful vibrations even through clothing,” Ayazi said.
The detection bandwidth is enormous - from broad, sweeping motions to inaudibly high-pitched tones. Thus, the sensor chip records all at once fine details of the heartbeat, waves the heart sends through the body, and respiration rates and lung sounds. It even tracks the wearer’s physical activities such as walking.
The signals are recorded in sync, potentially offering the big picture of a patient’s heart and lung health. For the study, the researchers successfully recorded a “gallop,” a faint third sound after the “lub-dub” of the heartbeat. Gallops are normally elusive clues of heart failure.
The researchers published their results in the journal npj Digital Medicine on February 12, 2020. The research was funded by the Georgia Research Alliance, the Defense Advanced Research Projects Agency (DARPA), the National Science Foundation, and the National Institutes of Health. Study coauthor Divya Gupta, M.D., a cardiologist at Emory University, collaborated in testing the chip on human participants.
Hermetically sealed vacuum
Medical research has tried to make better use of the body’s mechanical signals for decades but recording some – like waves traversing multiple tissues – has proven inconsistent, while others – like gallops – have relied upon clinician skills influenced by human error. The new chip produces high-resolution, quantified data that future research could match to pathologies in order to identify them.
“We are working already to collect significantly more data matched with pathologies. We envision algorithms in the future that may enable a broad array of clinical readings,” Ayazi said.
Though the chip’s main engineering principle is simple, making it work and then manufacturable took Ayazi’s lab ten years, mainly because of the Lilliputian scale of the gap between the silicon layers, i.e. electrodes. If the 2-millimeter by 2-millimeter sensor chip were expanded to the size of a football field, that air gap would be about an inch wide.
“That very thin gap separating the two electrodes cannot have any contact, not even by forces in the air in between the layers, so the whole sensor is hermetically sealed inside a vacuum cavity,” Ayazi said. “This makes for that ultralow signal noise and breadth of bandwidth that are unique.”
Detects through clothing
The researchers used a manufacturing process developed in Ayazi’s lab called the HARPSS+ platform (High Aspect Ratio Poly and Single Crystalline Silicon) for mass production, running off hand-sized sheets that were then cut into the tiny sensor chips. HARPSS+ is the first reported mass manufacturing process that achieves such consistently thin gaps, and it has enabled high-throughput manufacturing of many such advanced MEMS, or microelectromechanical systems.
The experimental device is currently battery-powered and uses a second chip called a signal-conditioning circuit to translate the sensor chip’s signals into patterned read-outs.
Three sensors or more could be inserted into a chest band that would triangulate health signals to locate their sources. Someday a device may pinpoint an emerging heart valve flaw by turbulence it produces in the bloodstream or identify a cancerous lesion by faint crackling sounds in a lung.
Here's how to subscribe to our free science and technology newsletter
Also read: Digital tool helps with tough COVID19 decision
These researchers co-authored the study: Pranav Gupta (first author), Mohammad Moghimi, Yaesuk Jeong and Omer Inan from Georgia Tech. The research was funded by the Georgia Research Alliance, the Defense Advanced Research Projects Agency (DARPA) Technology Office’s Advanced Inertial Micro Sensors program (contract # N66001-16-1-4064), and by the National Science Foundation/National Institutes of Health Smart and Connected Health Program (grant # R01 EB023808). The team’s work with human subjects was approved by Emory University and Georgia Institute of Technology Institutional Review Boards (IRB# H18248). Any findings, conclusions or recommendations are those of the authors and not necessarily of the sponsors.
Writer & Media Representative: Ben Brumfield (404-272-2780), email: ben.brumfield@comm.gatech.edu
Georgia Institute of Technology
Pagination
- Previous page
- 3 Page 3
- Next page